Is Stainless Steel a Element Compound or Mixture
Stainless steel is A A compound B A mixture C An element D An alloy Stainless steel is basically a alloy with aluminium Chromium and Carbon as elemen Grade Stainless steel is A A compound B A mixture C An element D. No stainless steel is a homogeneous mixture of iron and chromiumYES actually stainless steel is a compound because compounds are HOMOGENEOUS forms of matter.

Is Steel An Element Compound Or Mixture Answered Dear Learners
Matter may be a pure substance or it may be a mixture.

. This metal alloy can vary in composition. A mixture is a material that is made up of two more chemical compounds or substances that do not combine together chemically. It produces a thin layer of oxide on the surface of the steel.
The exterior part that we can handle is usually made of plastic. A mixture is not a compound. All of the above statements are true.
Classify stainless steel as an element compound heterogeneous mixture or homogeneous mixture. An intermetallic compound is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. It needs more than 1 elements to form a compound.
Find step-by-step Physical science solutions and your answer to the following textbook question. Salt stainless steel tap water sugar vanilla extract butter maple syrup aluminum ice milk cherry-flavored cough drops. However Stainless steel contains varying amounts of Carbon Silicon and Manganese also.
Steel is a mixture as its composed of two different elements ie. A mixture can contain both elements and compounds. A compound is a substance that is made up of two or more elements that combine chemically in a definite ratio of composition.
A compound contains two or more different atoms joined together. Sand and water Salt and Water Solutions Colloids etc. Mixtures may be composed of two or more elements two or more compounds or a combination of both.
A mixture contains two or more different substances that are only physically joined together not chemically. Hydrogen is a standalone element not a compound. Water Table Salt Carbon Monoxide etc.
Stainless steel is an alloy of Iron with a minimum of 105 Chromium. Steel cannot be considered element because it consists of more than 1 element and steel cannot be considered a compound because there is no chemical bond between the constituents. Hydrogen is not a compound.
So the clothes iron is not pure iron at all. An element is a pure substance that is made from a single type of atom. A compound is a substance that is composed of two or more different elements.
It can be stainless steel ceramic cast iron etc. The layer prevents corrosion. Classify air as an element compound heterogeneous mixture or homogeneous mixture.
Classify mercury in a thermometer as an element compound heterogeneous mixture or homogeneous mixture. A pure substance may either be an element or a compound. Represents an item composed of an element compound or mixture.
Stainless steel and cast iron are both iron alloys but they are not pure iron. Chromium serves the purpose of the passive layer. Hydrogen is not a compound since it only consists of its own atom.
Unlubricated behaviour of nitrided 316L stainless steel the effect wear tests with a sliding distance of 141 m were carried of layers formed on the surface and usage of the ni- out at room temperature 18 C relative humidity trided 316L stainless steel as implant material are still of about 50 sliding speed of 0078 m s1 normal. You can separate them by physical methods. A physical combination of two or more substances.
A mixture may be either homogeneous or heterogeneous. Classify the following as an element compound or mixture and justify your classifications. Iron and carbon that combine without chemical bond.
An element contains just one type of atom. Stainless steel is a group of ferrous alloys that contain a minimum of approximately 11 chromium a composition that prevents the iron from rusting and also provides heat-resistant propertiesDifferent types of stainless steel include the elements carbon from 003 to greater than 100 nitrogen aluminium silicon sulfur titanium nickel copper selenium niobium and. While the solid shiny part is usually a metal alloy.

Classify The Following As Pure Substances Or Mixtures Separate The Pure Substances Into Elements Compounds And Divide The Mixtures Into Homogeneous And Heterogeneous I Air Ii Milk Iii Graphite Iv Gasoline
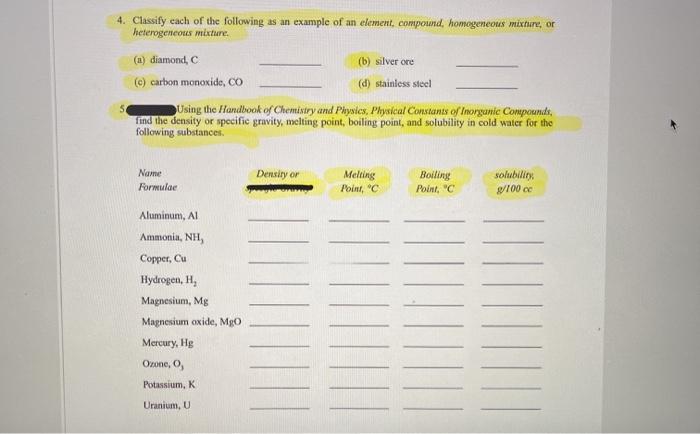
Solved 4 Classify Each Of The Following As An Example Of An Chegg Com

Classify The Following Into Elements Compounds And Mixtures A Sodium B Soil C Sugar Solution D Silver E Calcium Carbonate F Tin G Silicon H Coal I Air J Soap K Methane

Element Mixture Compound Activity Teachengineering

Pure Substances And Mixtures Acids Bases Quizizz

Solved 3 Classify Each Of The Following As An Element E Chegg Com

Is Gold An Element A Compound Or A Mixture Quora

3 5 Pure Substances And Mixtures Chemistry Libretexts
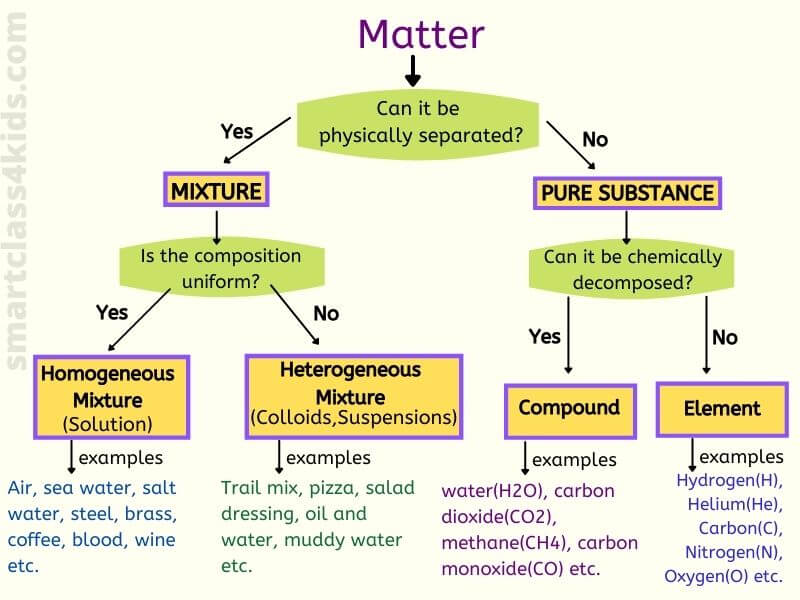
Explained Classifying Matter Elements Compounds Mixture

Chapter 22 24 Mixture A Combination Of Two Or More Substances In Which Each Substance Retains Its Properties Stainless Steel Mixture Of The Ppt Download

Solved 4 Classify Each Of The F Ollowing As An Example Of Chegg Com

What Is Stainless Steel Aperam

Match The Pairsstainless Steel Silverbhajani Mixturesaltcoalhydrogennon Brainly In

Is Steel An Element Compound Or Mixture Answered Dear Learners

Is Steel An Element Compound Or Mixture Answered Dear Learners

Classify The Following Substances As Element Compound And Mixtures Are Copper Hydrogen Bronze Brainly In

What Is Stainless Steel Aperam

Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube

Comments
Post a Comment